Introduction: Peripheral T-cell lymphoma (PTCL) comprises a heterogeneous group of rare neoplasms, the majority characterized by an aggressive course and short survival. Angioimmunoblastic T-cell lymphoma (AITL) is the second most common subtype (19%) of PTCLs, with generally poor long-term prognosis. The outcomes for most reported cases of AITL are derived from cohorts which include other PTCL subtypes. There is limited data for the role of autologous stem cell transplantation (ASCT) in AITL, and the associated predictive factors for prognosis. We present the largest single center cohort of AITL patients who underwent ASCT either upfront or at time of relapse.
Methods: We included consecutive patients with AITL who had confirmed diagnosis and underwent ASCT at our institution from May 2000 to November 2019. Primary endpoints: progression free survival (PFS) and overall survival (OS). Secondary endpoints were cumulative incidence of relapse (CIR), non-relapse mortality (NRM), and to identify prognostic factors associated with PFS and OS. Kaplan-Meier method was used to estimate OS and PFS. CIR and NRM were determined using the competing risks method. Cox regression analyses were used to determine prognostic factors.
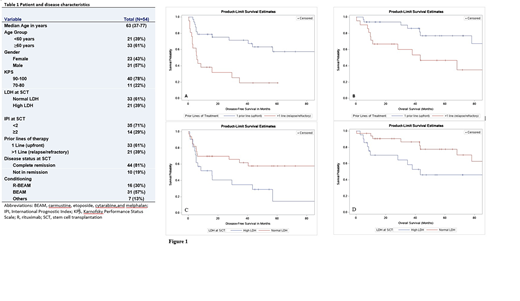
Results: The study included 54 patients with a median age of 63 (range, 37-77) years and male predominance (57%). All patients had advanced stage III/IV at diagnosis. Additional patient and disease characteristics are outlined in Table 1. Carmustine, etoposide, cytarabine, and melphalan (BEAM) with (30%) or without (57%) rituximab (R; used at the discretion of treating physician for EBER-positive AITL) were the most commonly used preparative regimens. With a median follow-up of 47.4 (range, 7.1-142.2) months, the median PFS and OS of all study patients were 41 and 108 months, respectively. The 2- and 4-year PFS/OS were 58%/83% and 46%/65%, respectively. CIR of relapse at 1, 2, and 4 years were 30%, 34%, and 44%, respectively. NRM at 1, 2, and 4 years were 7.5%, 7.5%, and 10%, respectively. All variables listed in Table 1 were assessed for their prognostic impact in univariate analysis (UVA) for PFS and OS. Of those, transplant for relapsed AITL (HR 3.716 95% CI: 1.728-7.991; p=0.0008) and high LDH at transplant (HR 2.139, 95% CI: 1.023-4.471; p=0.0433) were significantly associated with worse PFS (Figure 1A, C). There was a tendency for improved PFS for women (HR 0.56, 95% CI: 0.259-1.209; p=0.1398) and patients who received R-BEAM conditioning (HR for BEAM 1.99, 95% CI: 0.808-4.899; p=0.1344). Similar UVA results were noted for OS, where transplant for relapsed AITL (HR 2.943, 95% CI: 1.173-7.382; p=0.0214) and high LDH at transplant (HR 2.771, 95% CI: 1.076-7.138; p=0.0348) were significantly associated with worse OS (Figure 1B, D). On multivariable analysis (MVA), transplant at relapse (HR 3.716 95% CI: 1.728-7.991; p=0.0008) was associated with inferior PFS (HR 3.038, 95% CI: 1.386-6.659; p=0.0055) and OS (HR 2.291, 95% CI: 1.054-4.979; p=0.0364). High LDH at transplant was associated with worse PFS (HR 2.291, 95% CI: 1.054-4.979; p=0.0364), and with a trend for inferior OS (HR 2.259, 95% CI: 0.838-6.093; p=0.1073). Only 10 (19%) patients were transplanted with active disease at transplant; disease status at transplant didn't have a significant impact on outcomes in UVA and MVA. A subset analysis subgrouping patients by 1 (n=33) vs 2 (n=16) vs >2 (n=5) prior lines of therapy showed no significant difference in outcomes between 2 vs >2 prior lines of therapy.
Conclusions: Upfront ASCT is associated with significantly improved and durable survival in patients with AITL. Receiving more than one prior line of therapy (transplant for relapsed AITL) and elevated LDH at transplant are associated with very poor prognosis, for which allogeneic transplant and alternative novel therapies should be further explored.
Nieto:Secura Bio: Other: Grant Support; Astra Zeneca: Other: Grant Support; Novartis: Other: Grant Support; Affimed: Consultancy, Other: Grant Support. Popat:Bayer: Research Funding; Novartis: Research Funding. Qazilbash:Angiocrine: Research Funding; Janssen: Research Funding; Bioline: Research Funding; Amgen: Research Funding; Bioclinica: Consultancy. Shpall:Takeda: Other: Licensing Agreement; Novartis: Membership on an entity's Board of Directors or advisory committees; Magenta: Membership on an entity's Board of Directors or advisory committees; Zelluna: Membership on an entity's Board of Directors or advisory committees; Adaptimmune: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Flowers:Cancer Prevention and Research Institute of Texas: Research Funding; Karyopharm: Consultancy; Pharmacyclics/Janssen: Consultancy; Eastern Cooperative Oncology Group: Research Funding; Burroughs Wellcome Fund: Research Funding; TG Therapeutics: Research Funding; Millennium/Takeda: Consultancy, Research Funding; Acerta: Research Funding; Spectrum: Consultancy; National Cancer Institute: Research Funding; V Foundation: Research Funding; Kite: Research Funding; Bayer: Consultancy; Leukemia and Lymphoma Society: Membership on an entity's Board of Directors or advisory committees; Genentech, Inc./F. Hoffmann-La Roche Ltd: Consultancy, Research Funding; Denovo Biopharma: Consultancy; Celgene: Consultancy, Research Funding; BeiGene: Consultancy; AbbVie: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; OptumRx: Consultancy. Champlin:Johnson and Johnson: Consultancy; DKMS America: Membership on an entity's Board of Directors or advisory committees; Genzyme: Speakers Bureau; Cytonus: Consultancy; Omeros: Consultancy; Takeda: Patents & Royalties; Actinium: Consultancy. Hosing:NKARTA Inc.: Consultancy. Khouri:Bristol Myers Squibb: Research Funding; Pfizer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.